PS1.A: Structure and Properties of Matter
|
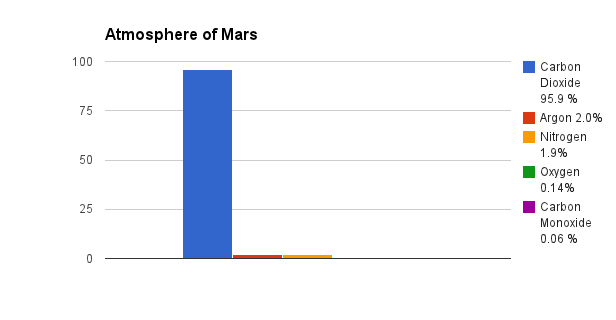
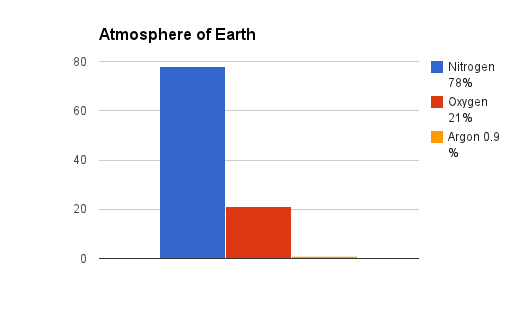
 On Earth gasses are usually nonmetal elements, or compounds of nonmetals. Earth's atmosphere is made up primarily of Nitrogen (N2), Oxygen (O2), and Argon (Ar). The vast majority of our atmosphere is Nitrogen. According to NASA, the atmosphere of Mars is mostly Carbon Dioxide (CO2), Argon (Ar), Nitrogen (N2), Oxygen (O2), and Carbon Monoxide (CO).
On Earth gasses are usually nonmetal elements, or compounds of nonmetals. Earth's atmosphere is made up primarily of Nitrogen (N2), Oxygen (O2), and Argon (Ar). The vast majority of our atmosphere is Nitrogen. According to NASA, the atmosphere of Mars is mostly Carbon Dioxide (CO2), Argon (Ar), Nitrogen (N2), Oxygen (O2), and Carbon Monoxide (CO).
 For the law, Pressure is represented by P. Temperature (in Kelvin) is T. The number of moles of gas is represented as n. Volume is represented as V. Lastly the equation has a constant R, which is only a number used to make the units work out. It is similar to a power adaptor for electronic devices. If different units for Pressure or Volume are used, a different R is applied. For our purposes, we will be working with atmospheres, and Liters, so R will be 0.08206 L×atm / mol×K.
For the law, Pressure is represented by P. Temperature (in Kelvin) is T. The number of moles of gas is represented as n. Volume is represented as V. Lastly the equation has a constant R, which is only a number used to make the units work out. It is similar to a power adaptor for electronic devices. If different units for Pressure or Volume are used, a different R is applied. For our purposes, we will be working with atmospheres, and Liters, so R will be 0.08206 L×atm / mol×K.
| CCSS.ELA-Literacy.RST.9-10.3 Follow precisely a complex multistep procedure when carrying out experiments, taking measurements, or performing technical tasks, attending to special cases or exceptions defined in the text. |